International collaborative researchNP-PBRC Applied
Plasma Medicine Center (APMC)
International
collaborative research
Commercialization and global marketing for
plasma medical devices

-
Affiliation of Leibniz Institute
Leibniz - INP


- Experience of development about
medical devices using atmospheric
pressure plasma and standardization of DIN 
- Possession of technology about
electromagnetic stability for plasma
medical devices which can apply to the
medical field 
- Public certification for clinical trial
about skin disease of medical devices
using atmospheric pressure plasma 
-
Passing through clinical studies of
plasma medical devices
<Develolopment Procedure>
· Performance evaluation
· Preclinical study/
clinical study
· FDA, CE certification

- Technology
- Certification
- Clinical Trial
-
Affiliation of Kwangwoon Univ.
PBRC


- Commercialization of medical devices
using atmospheric pressure plasma 
- Joint research for international
standardization of medical devices
using atmospheric pressure plasma 
- Clinical trial progress which is based
on the domestic hospital network 
-
Acquisition of core tech and establishment of
international standard of
AP plasma medical devices.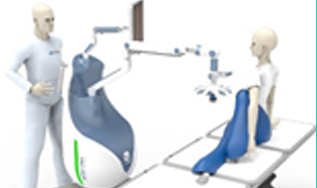
<Commercialization procedure>
· Development protocol
· Core tech dev.
· Medical device dev.
· Proto type
· Device standardization 
The role of PBRC Kwangwoon Univ.
- To lead AP plasma medical device development for human skin disease treatment
- to set up a diagnosis system for electro-optical characteristics and radicals of AP plasma medical devices.
- to establish the Joint Test facilities for clinical ability test in a joint
- to stablish and manages the education program and training center
- to develop technology for commercialization of collaborative research outcomes
- to obtain Medical device certificate in Korea and support clinical trials for kINPEN commercialization
The role of INP, Germany
- To share the clinical data of AP plasma medical devices of INP and Establish a venture company in Korea to bridge Asian market.
- to execute Collaborative research and support for efficacy test of AP plasma devices for skin disease AP plasma
- to share PBRC research on AP plasma devices and applying them to biological characteristics.
- to study mechanism on skin disease treatment
- to function as a collaborative research center for education and training human resources.
| Division | Research goal | 1st stage (2016 – 2017) |
2nd stage (2018 – 2021) |
|---|---|---|---|
| Final goal |
Development and international standardization of AP plasma for skin disease treatment |
Development of proto-type AP plasma medical devices and skin disease efficacy evaluation |
Commercialization and international standardization of AP plasma devices and its treatment mechanism for skin diseases |
| Original tech. elements |
Efficacy study on AP plasma medical device for skin diseases and its mechanism study |
 |
|
 |
|||
 |
|||
 |
|||
 |
|||
| Original technology development, commercialization and international standardization for AP plasma medical devices |
 |
||
 |
|||
 |
|||
Development of plasma sources and Study of their characteristics
- Human skin applicable AP plasma source development : Jet and plane electrode structure
- Study of physical characteristics of AP plasma sources
Bio-medical efficacy study of AP plasma on skin diseases.
- Efficacy study on skin diseases such as atopy, acne, hair loss, aging and skin cancer
- Clinical evaluation of Plasma treatment by using skin disease models
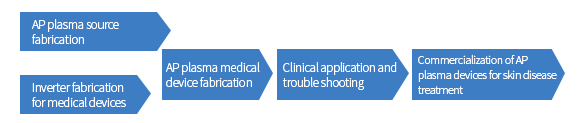
Development, commercialization and establishment of
international standard of AP plasma devices for clinical application
- Commercialization and international standardization of AP plasma skin disease treatment devices
- Efficacy study of skin disease and skin care, and device commercialization by clinical trials


